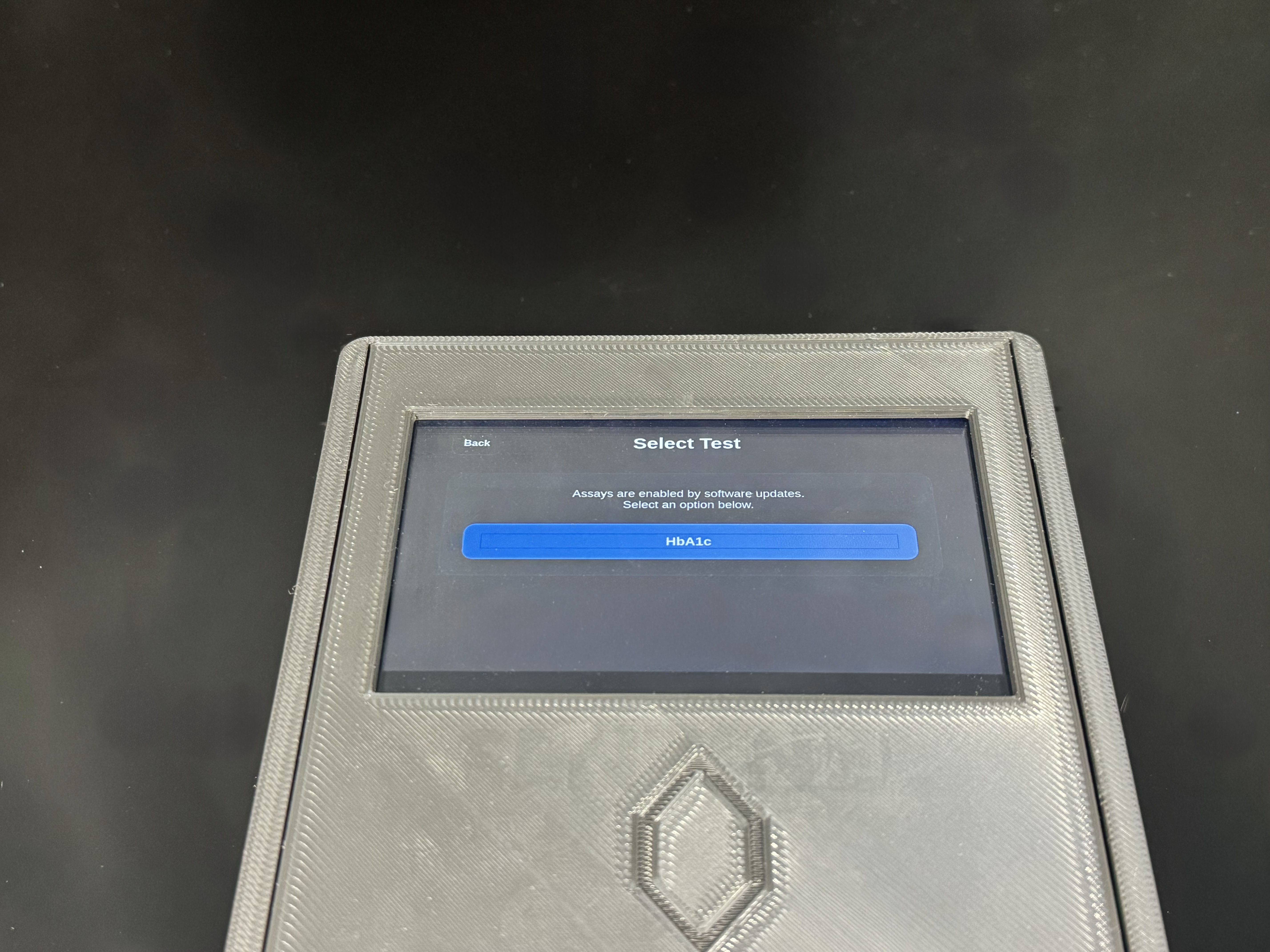
Scalable test menu
A consumable driven architecture designed to support additional assays overr time.
Business Model: Razor & Blade
Corvidan follows a razor and blade model built around a durable, professional use measurement reader and single use electrochemical test strips. The reader is placed once, while consumables drive recurring usage over time.
Each test strip is assay specific and developed independently, allowing new measurements to be introduced without modifying the core device hardware. This structure supports predictable manufacturing, controlled expansion and repeatable workflows.
By separating instrumentation from consumables, Corvidan aligns long term platform adoption with recurring revenue. All while maintaining consistent measurement across use cases.
Engineering Development
2025
Analytical Bench Evaluation
Q4 2025
Workflow Validation
Q2 2026
Limited Evaluation Deployment
Q4 2026
Regulatory Preparation Milestone
Q1 2027

